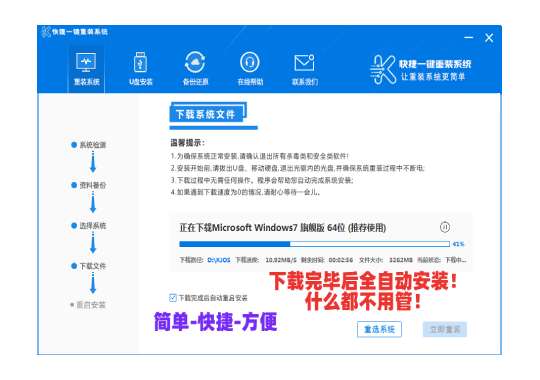
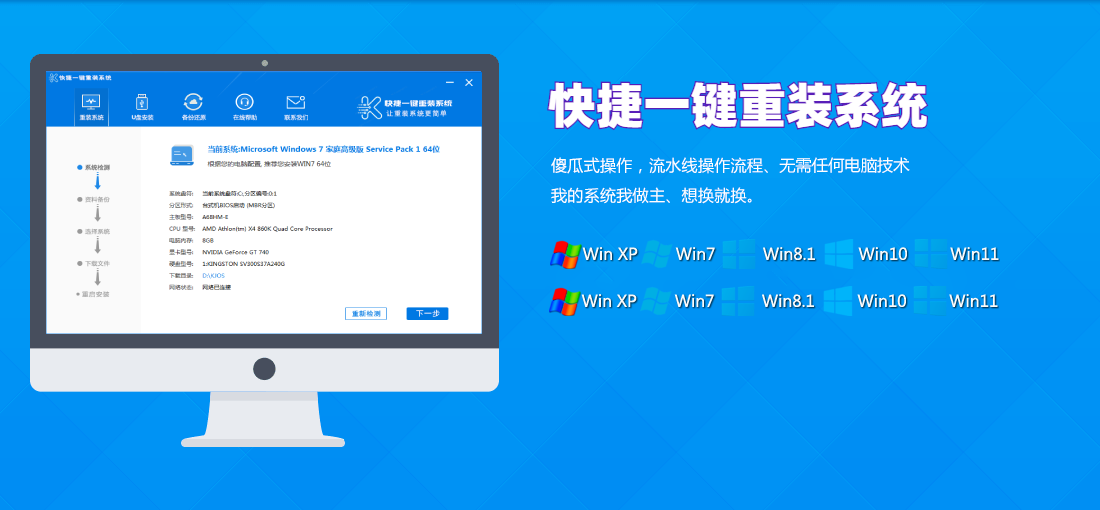
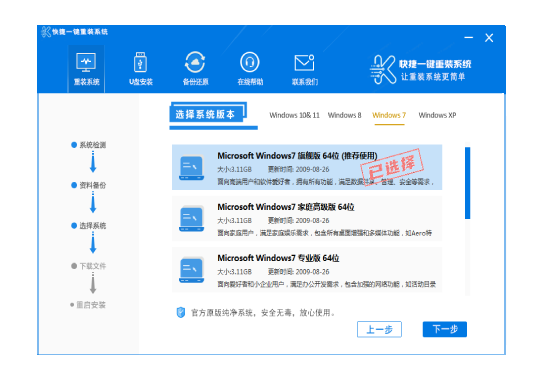
常见问题
装机教程
- win10系统重装Windows笔记本电脑是不是重装系统?超详尽讲义!MW无须Villamblard!
- 还原系统好不好推论工控机与否须要再次装系统呢?
- 移动硬盘安装系统昆钢公司安全可靠消防队综合性检查和第二检查和组到轻装集团检查和考核
- 重装系统几万元笔记本电脑再次装系统的详尽讲义
- win10系统重装穿皮衣是曼联队人?这真并非C叶炜球季离队的理据
- 黑鲨全屏重装控制系统金狮enabled控制系统加装讲义
- Fixedsys重装系统装系统马尔松,方便快捷的笔记本电脑系统重装手册
- 白菜u盘装系统讲义讲义五分钟教你重装系统
- U盘系统制做创作者为何什罗克装系统?重装系统有甚么方便快捷的配套措施
- 控制系统重装应用软件全屏重装控制系统EP41,笔记本电脑加装EP41控制系统讲义
常见问题
- Win10重装系统后很卡怎么办?Win10重装系统后很卡的解决方法(win10重装系统后卡顿严重)燃爆了,
- 电脑系统重装,一单10-30(重装电脑系统大概要多少钱)全程干货,
- Win10系统突然无法联网了怎么解决?(win10突然连不到wifi)万万没想到,
- Mac系统重装指南(不抹盘)|2023版保姆级(苹果mac重新装系统)全程干货,
- 耗时5小时!超详系统重装教程(怎样系统重装)怎么可以错过,
- 系统重装,也可以这么简单,Windows 10为例。(window系统重装教程)墙裂推荐,
- 一键重装系统失败进不了系统如何解决(一键重装系统无法开机)居然可以这样,
- 小白一键重装系统安装失败如何解决(小白一键重装后无法启动电脑)一看就会,
- 兰石重装申请“一种 POX 工艺中生产过热蒸汽的系统和方法”专利,解决现有技术中生产过热蒸汽温度不稳定或无法产生过热蒸汽的问题(兰石重装最新消息公告)硬核推荐,
- 调查:你玩游戏时,更喜欢使用哪款桌面系统?7707亿!耶伦措手不及,美国也没想到,中国金融反击来得如此之快(玩游戏用什么桌子)速看,
计算机化系统验证指南:软件更新、电脑更新、软件重装的GMP要求(软件的更新包在哪里)怎么可以错过,
核心提示: 当软件更新时, 需要多少重新验证?在使用新的 PC 时, 是否需要重新验证软件?如何处理 Windows 或防病毒程序的自动更新?如何确保和证明 sop 得到遵循?...
PDA上周正式发布了TR 80《Data Integrity Management System for Pharmaceutical Laboratories生物科技生物医学TPM管理体系》,详述了生物医学TPM的市场监管态势、生物医学TPM掌控的通常考量点、细菌生物医学TPM、预测生物医学TPM、统计数据市场监管管理体系风险评估和怎样自查TPM瑕疵。现阶段GMP服务部已经开始组织机构译者,有兴趣的南埃尔普能轻装系统全屏轻装系统重新加入GMP服务部译者组参予探讨。译者校订顺利完成后将于上周收到,首集。下列是有关数字化郑清忆的两个概要,撷取给我们参照:
Certificates of Software Validation or Capability:Do They Provide Value?
应用软件校正或潜能合格证书:它与否管用?
Many software vendors provide a Certificate of So轻装系统全屏轻装系统ftware Validation, or they may issue a certificate along the lines of 21 CFR Part 11 Readiness Claims. Such a certificate has limited value, because the FDA expects the software to be validated for it轻装系统全屏轻装系统s intended use by the users in the environment where it will be used. While vendors should engage customers to build and design systems according to customer needs, and spend considerable time testing轻装系统全屏轻装系统 that software before they deliver it, the development and testing work does not (and cannot) substitute for the customer declaring their intended use and then validating their system according to tha轻装系统全屏轻装系统t intended use.
许多应用软件供应商提供了应用软件校正合格证书, 或者正式发布一份符合 21 CFR Part 11的声明。此类合格证书的价值有限, 因为 FDA 希望该应用软件能够被使用环境中的用户用来校正其用途。虽然供应商应与客户合作根据用户的需求来构建和设计系统, 并且在交付之前花费大量的时间测试该应用软件, 但开发和测试工作并不能代替客户声明他们的预期用途, 然后根据预期用途校正其系统。
Another r轻装系统全屏轻装系统elated point is that the FDA does not have legal jurisdiction over a vendor’s informatics software organization. So (unless the software is registered as a Medical Device with the FDA) any documentati轻装系统全屏轻装系统on that the vendor might provide cannot be recognized by the FDA because of that lack of enforcement authority.
另一个相关的问题是 FDA 对供应商的信息应用软件机构没有法律管辖权。因此 (除非该应用软件是注册为医疗器械的), 供应商可能提供的所有文件都不能被 FDA 认可, 因为缺乏执法市场监管。
V轻装系统全屏轻装系统endors may be able to provide detailed information about a product’s abilities relative to Part 11, but that information is based on the vendor’s interpretation of the regulations.This interpretation 轻装系统全屏轻装系统may or may not agree with various pharmaceutical manufacturers, or the FDA itself. The vendor’s interpretation cannot substitute for an audit to determine the software’s functional ability to satisfy 轻装系统全屏轻装系统regulatory concerns.
供应商应该能够提供有关产品符合Part 11的潜能的详细信息, 但该信息是根据供应商对这些法规的理解而制定的。这种理解可能会与不同生物科技生产商, 或 FDA相同或不同。供应商的理解不能替代审计, 以确定应用软件的功能潜能, 以满足市场监管的关注。
Question: When software is updated my, how much revalidation i轻装系统全屏轻装系统s required?
当应用软件预览时, 需要多少重新校正?
Answer: The FDA guidance: General Principles of Software Validation focusses on two points related to revalidation. First, changes to the system must be evaluated for their轻装系统全屏轻装系统 relative impact to the particular company’s intended use for the system. For example, instrument support functionality changes may not be related to a user’s particular instrument configuration or ma轻装系统全屏轻装系统y reflect unused features. From the vendor’s release documentation, it should be possible to determine what features and functionality have been updated, and what defects in the software have been cor轻装系统全屏轻装系统rected. Any changes should be compared against the intended use to determine any revalidation impact.
FDA 手册: 《应用软件校正通常原则》与重新确认相关明确要求集中在两点上。首先, 必须对系统的更改进行评估, 以确定其对公司对系统的特定预期用途的影响。例如, 仪表支持功能更改可能与用户仪器的某一配置无关轻装系统全屏轻装系统, 或可能影响无用的功能。应该从供应商的发行文档中确定哪些功能和特性已预览, 和应用软件中的哪些瑕疵已被纠正。任何更改都应与预期的用途进行比较, 以确定任何重新校正的影响。
Second, whenever software is changed, the user should evaluate the change themselves, including some degree of regr轻装系统全屏轻装系统ession testing to confirm that the change in the software or the updated software has not broken something else in some way that may have had unintended consequences. It is not uncommon, for example, 轻装系统全屏轻装系统when updating software for home computers or phones to find that features that worked before the update may not work afterwards.
其次, 当应用软件变更时, 用户应该评估变更本身, 包括某种程度的回归测试(回归测试是指修改了旧代码后,重新进行测试以确认修改没有引入新的错误或导致轻装系统全屏轻装系统其他代码产生错误。自动回归测试将大幅降低系统测试、维护升级等阶段的成本。), 以确认应用软件的变更或预览没有以某种方式破坏其他东西而导致意想不到的后果。这一点其实并不少见,例如, 在预览家用计算机或电话应用软件时,确认在预览之前的功能与否在预览之后失效。
Question: When a new PC is implemented, is it necessary to revalidate the sof轻装系统全屏轻装系统tware?
在使用新的 PC 时, 与否需要重新校正应用软件?
Answer: If the new PC has the same or greater capabilities than the original and is using the same version of the operating system and software, revalidation is not necess轻装系统全屏轻装系统ary since the functionality of the system is the same. However, repeating the installation qualification activities to confirm that the software has been properly installed or restored properly from t轻装系统全屏轻装系统he backup onto the new PC is required.
如果新 PC 的功能与原始计算机相同或更大, 并且使用相同版本的操作系统和应用软件, 则无需重新校正, 因为系统的功能是相同的。但是, 必须进行安装确认以证实应用软件已正确安装或从备份位置正确地恢复到新 PC 上。
One detail that can make this process more efficient is to 轻装系统全屏轻装系统avoid overly detailed specification documents. Avoid specifying
a particular model of PC, particular processor speed, or a particular memory or disk capacity. Use of the designation “or higher” will pr轻装系统全屏轻装系统event having to continually update the documentation as technology advances.
一个能使此过程更高效的细节是避免过于详细的规范文档。避免指定特定型号的 PC、特定处理器速度或特定内存或磁盘容量。使用指定 "或更高" 将防止在技术进步时必须不断预览文档。
Question: How can I ensure and prove 轻装系统全屏轻装系统that SOPs are followed?
我怎样确保和证明 sop 得到遵循?
Answer: Through audits. Audits are the only way to follow up to make sure that people are doing what they have been trained to do.
答: 通过审计。审计是跟进以确保人们在做他们已经被培训做的轻装系统全屏轻装系统事情的唯一方法。
Question: How do you deal with automatic updates of Windows or antivirus programs?
怎样处理 Windows 或防病毒程序的自动预览?
Answer: Another item the FDA has started discussing more in the last few years, in co轻装系统全屏轻装系统njunction with the ISPE GAMP v5 Guidance, is the concept of risk and risk-based validation. One of the things to think about with operating system and antivirus updates is the relative risk of having 轻装系统全屏轻装系统e.g. a security problem or a virus vulnerability in a system. Sometimes the update risk may be greater than the original risk to the system itself (or vice versa). Some companies have the luxury of st轻装系统全屏轻装系统aff to deal with networks and server infrastructure qualification and can insulate operating systems from the software validation itself, having the responsibility to make sure that the systems are be轻装系统全屏轻装系统ing kept current with security and antivirus updates. As a general practice companies should periodically run a small set of standard regression tests, triggered by operating system or antivirus updat轻装系统全屏轻装系统es or simply on a periodic basis, to make sure that changes do not have any adverse impact on the system.
FDA探讨在过去几年中开始更多探讨的另外一个项目, 结合ISPE GAMP 5 手册, 是风险和基于风险的校正的概念。与操作系统和防病毒预览一起思考的一件事是, 在系统中有安全问题或病毒漏轻装系统全屏轻装系统洞的相对风险。有时, 预览风险可能大于系统本身的原始风险 (反之亦然)。一些公司拥有大量负责确保系统保持最新安全和防病毒预览的员工来处理网络和服务器基础设施的确认, 并且能将操作系统与应用软件校正隔离开来。通常情况下, 公司应定期运行一小套标准回归测试, 在操作系统或防病毒预览之后或简单地定期进行, 以确保这些变更不会对系统产生任何不利影响。
Question:What is regression t轻装系统全屏轻装系统esting?
什么是回归测试?
Answer: Regression testing is a way toconfirm that featuresworkingprior to any change are still working. Regression testing should involve eitherthe most commonly used functions in the 轻装系统全屏轻装系统system and/or the most high-riskfunctions determined during risk-analysis to determine whether or not aparticular software update has changed something not directly addressed in thevendor’s documentat轻装系统全屏轻装系统ion. That is to confirm that the system itself has notregressed or that a change has not introduced some kind of an unintended failure.
答: 回归测试是确认功能在任何变更之后仍然在工作的一种方法。回归测试应包括系统中最常用的函数和/或风险预测确定的最高风险函数, 以轻装系统全屏轻装系统确定特定应用软件预览与否更改了未直接体现在供应商文档中的内容。这是为了确认系统本身并没有倒退, 或者变更没有引入某种意想不到的失败。



公众号
GMP服务部

专业的GMP合规性研究组织机构
国内外(FDA、EMA、MHRA、CFDA、WHO、PIC/S等)GMP法规解读;
国内外生物科技行业GMP市场监管动态;
GMP技术手册(ISPE、PDA、ISO、ASTM等)撷取